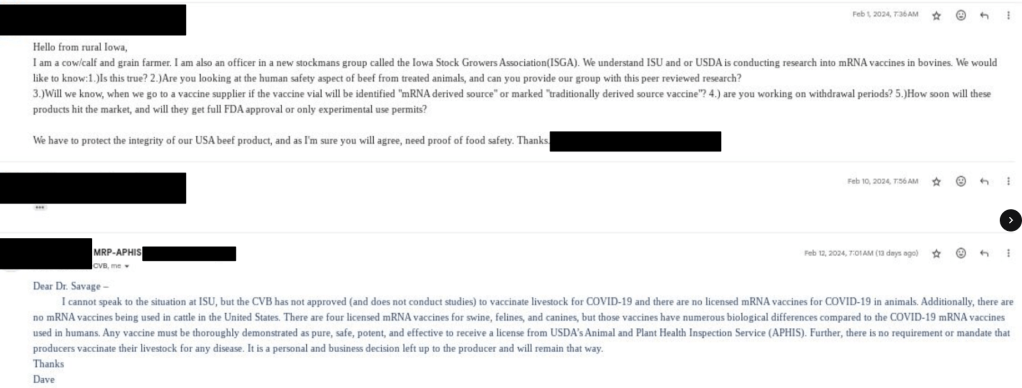
Letters Sent by “Fans” of our Work!
Exhibit A & B of how the USDA treats farmers who don’t go along to get along.
Mr. and Mrs. “Salvage”
Exhibit A

The Food Security Act of 1985, as amended, requires any person who produces an agricultural crop on Highly Erodible Land (HEL) to be actively applying an approved conservation plan or conservation system in order to be eligible for certain United States Department of Agriculture (USDA) program benefits, as set forth in the USDA regulation, 7 CFR, Part 12, 12.4.
You were notified that the Natural Resource Conservation Service (NRCS) would conduct a conservation compliance status review on Farm #942, Tract #1463, in Ringgold County, Iowa. I have made a Preliminary Technical Determination that you are Not Actively Applying an Approved Conservation Plan or Conservation System (NA) on Tract #1463 because of the following reason(s): By signing Farm Service Agency form 1026, you grant USDA personal access to your farm for the purpose of USDA completing required activities such as compliance status reviews. NRCS received a request from you stating that you did not want NRCS to complete a required status review in 2012. NRCS understands this to be “denied access”. Denying access results in a determination of Not Actively Applying an Approved Conservation Plan or Conservation System (NFSAM 520.6).
This preliminary determination will become final 30 days after receipt of this letter unless you request one of the following options in writing: 1) A reconsideration and field visit. During the field visit we will review the NRCS basis for our determination, answer any questions you have regarding this preliminary determination, and offer an opportunity for you to provide additional information regarding this determination.